Science
Clinical evidence
In 2013, our scientific founders discovered that fat grafting enriched with a high concentration of the patient’s own (autologous) ex-vivo expanded mesenchymal stems/stromal cells isolated from adipose tissue (“MSCs”), delivered a far superior result compared to non-enriched fat graft i.e., pure fat graft (Lancet, 2013; Stem Cells Translational Medicine, 2019).
Since then, StemMedical has developed a method to isolate a small number of MSCs from the patient’s adipose tissue (i.e., fat) and subsequently expand the MSCs safely and efficiently in our manufacturing facility to sufficiently boost the number of stem cells needed to achieve the desired concentrations.
The ability to isolate and expand the MSCs conveys unique benefits in plastic surgery where survival of transplanted fat is critical for the end result. Specifically the process allows us to significantly increase the concentration of a patient’s own MSCs in fat compared with that achieved by conventional and cell-assisted lipofilling.
Whereas conventional lipofilling yields 10,000-30,000 MSCs per ml of fat, and cell-assisted lipofilling offers a slightly higher concentration, our plastic surgery product Stemform provides a minimum concentration of 20 million MSCs per ml of fat for our cosmetic treatments.
This concentration ensures a reliable fat graft when mixed with the patient’s own freshly harvested adipose tissue as per Stemform procedures. This is demonstrated under strict scientific rigor and principles as documented in our published findings . We are not familiar with any other method using the patient’s own cells and tissue capable of producing similar outcomes.
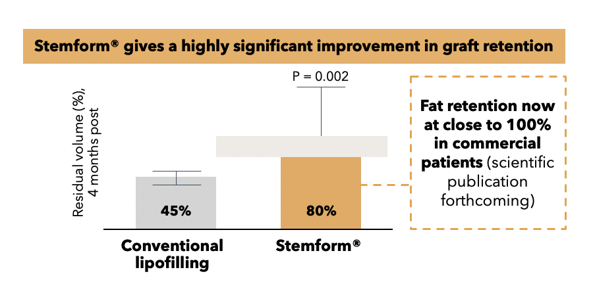
Thus, a patient who receives the Stemform treatment can obtain a predictable and natural aesthetic result regardless of whether they are seeking breast augmentation, facial filling and/or skin rejuvenation. This method provides patients a reliable and permanent natural alternative to temporary and artificial/synthetic solutions, such as breast implants and various hyaluronic acid facial fillers and toxins.
StemMedical has set out to scale its cell treatments that dramatically improve a patient’s quality of life. Clinical studies have shown that MSCs hold the potential to treat various musculoskeletal, respiratory, cardiovascular, and gastrointestinal disorders such as osteoarthritis, asthma, ischemia-reperfusion injury, inflammatory bowel diseases, type 1 diabetes, and chronic wounds.
Osteoarthritis (OA) of the knee, with more than 300 million patients worldwide, has shown promising clinical results with the use of MSCs and the StemMedical platform is uniquely suited to deliver a differentiated product in OA. Following careful analyses, StemMedical has initiated the first clinical development program for treatment of knee OA, with a clinical proof of concept trial planned for 2024.