Why invest
Our story
Platform
StemMedical’s core manufacturing competencies are focused on producing and expanding very high quantities of potent and affordable MSCs.
StemMedical can produce and harvest 12-16 billion ASCs from 100 ml of adipose tissue over 2 weeks using a scalable 3D cell culturing process instead of a manual non-scalable 2D cell process over several more weeks (as is currently industry standard).
Production takes place at our production facility outside Copenhagen. To expand capacity, blueprints have been made for a 2,500 sqm manufacturing site, designed for Good Manufacturing Practices (GMP) readiness, and with a planned capacity of over 7,000 batches per year. Work is currently in preparatory stage.
See more information about our platform here

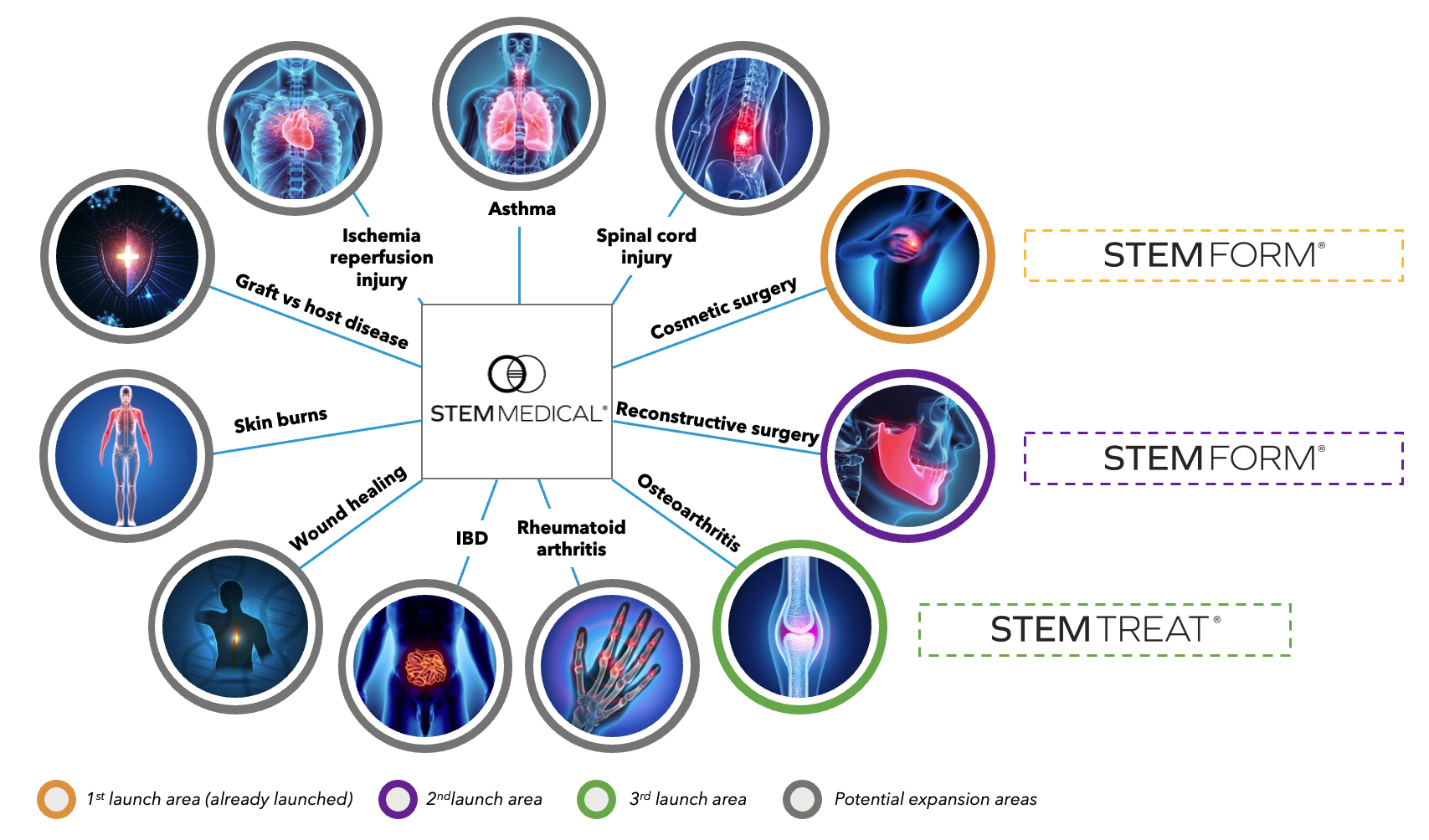
Broad application potential
The StemMedical MSC manufacturing platform has multiple potential applications.
Our most mature branch and application of our platform is in Plastic Surgery. Here we focus on cosmetic and reconstructive surgery and is commercially launched for cosmetic applications in Denmark with launches for UK and Norway coming in H2 2023.
Our second branch and application of our platform is in Therapeutics, where we are focusing on osteoarthritis of the knee. We are currently at the pre-clinical stage and will be starting our phase 1 clinical trial in 2024.
Fact sheet
Commercial patients treated as of October 2023: 67
Employees in the company: 20
PhDs in senior management: 6
Big pharma professionals in senior management: 3
MSCs produced per patient per batch: 12-16 billion
Manufacturing time: 15 days
Transport validation: 48 hours
Programs in clinical development: 1
(Osteoarthritis of the knee)