Osteoarthritis
Osteoarthritis (OA) is a degenerative disease characterized by severe joint pain and degradation of the cartilage as well as loss of chondrocyte function. Osteoarthritis is the most frequent cause of disability in older adults and is most commonly found in the knees, hips and hands. 10-13% of all individuals over 60 years of age have OA, and an aging population and increasing prevalence of obesity means that OA is on the rise. In Europe alone, the costs of OA on society are estimated to >400 billion €/year (approximately 11.000 €/patient/year, direct and indirect costs included).
Knee OA is globally affecting ~23% of individuals aged 40 or over, corresponding to approximately 650 million people worldwide, and it is considered the 11th highest contributor to global disability measured in years of life lived with disability in 2010.
Current standard of care focus on pain relief and suppressing inflammation, but only offers short term relief and does not stop or reverse progression, nor treat the underlying cartilage degradation. Due to the chronic nature of knee OA, total knee arthroplasty is usually indicated when above treatments fail to control the knee pain.
Stemtreat consists of ex-vivo expanded mesenchymal stem cells derived from MSC(AT) used as autologous or allogenic treatment for patients with knee OA. Following ex-vivo culturing, the Stemtreat product is mixed with freshly harvested fat (via liposuction) and injected into the knee.
Fat
MSCs
MSC(AT)s belong to the group of mesenchymal stem/stromal cells (MSCs), which are fibroblast-like adult cells. The MSCs have a high proliferation potential and the ability to differentiate into adipose tissue, bone, cartilage, tendon, skin and muscle. They are also considered being involved in local inflammation homeostasis and tissue replenishment.
Therefore, MSCs are hypothesized to enter the deteriorated cartilage area creating a regenerative microenvironment promoting cartilage regeneration. In addition, MSCs are thought to modulate/suppress the inflammatory state in the joint resulting in reduced pain and delayed disease progression.
So far, clinical studies have shown the potential of MSCs in treating OA, both regarding the treatment’s safety and efficacy. Clinical studies have reported significant effects of intraarticular MSC(AT) injections both improving functional and physical scores, and cartilage repair.
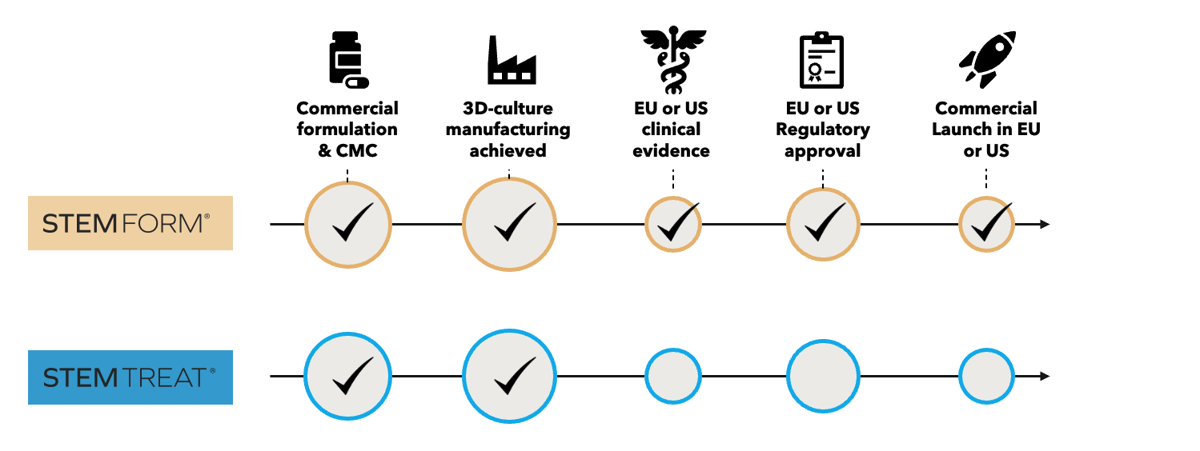
Stemtreat is manufactured in collaboration with Odense University Hospital. Our extensive experience with the manufacturing of our Stemform product since 2019. This allows StemMedical to quickly scale up the manufacturing process for Stemtreat and investigate the use for osteoarthritis of the knee and more indications in the future.
The Stemtreat manufacturing process starts with lipoaspirate, obtained by liposuction procedure, and followed by ex-vivo expansion culture to obtain high amounts of mesenchymal stem cells derived from adipose tissue (MSC(AT)s).
”A lot of the complexity with gene therapy is in product-related issues, not the clinical issues. Whereas with normal drug review, I'd say 80% is the clinical portion and 20% is the CMC and product portion of the review. I think with gene therapy and cell-based regenerative medicine, it's completely inverted."
A clinical trial application for a phase 1 exploratory clinical study with Stemtreat is under review and we aim to initiate this study in 2024. Following successful study read-out, a randomized, placebo controlled, double-blinded phase 1/2 clinical trial will follow to investigate the safety and efficacy of the STEM-OA product in subjects with mild to moderate knee OA. The phase 1/2 clinical
trial will create the basis for a larger phase 2/3 trial to collect data for a global regulatory approval. Stemtreat will investigated as an autologous (the patient’s own cells), and an allogeneic treatment (cells from a donor).
Reference: clinicaltrials.gov