Intellectual property
There are substantial barriers to entry for stem cell therapies, and technical know-how is significant given the deep competencies required across cell biology, bioengineering, manufacturing, regulatory affairs, and their application in plastic surgery. Furthermore, cell manufacturers are mostly equipped to produce large batches of allogenic cells or small batches of autologous cells but rarely set up to produce large batches of autologous cells. The technical challenges of producing 10-20 billion potent autologous MSCs to reach a high dose of ≥20 million MSCs/ml adipose tissue are significant.
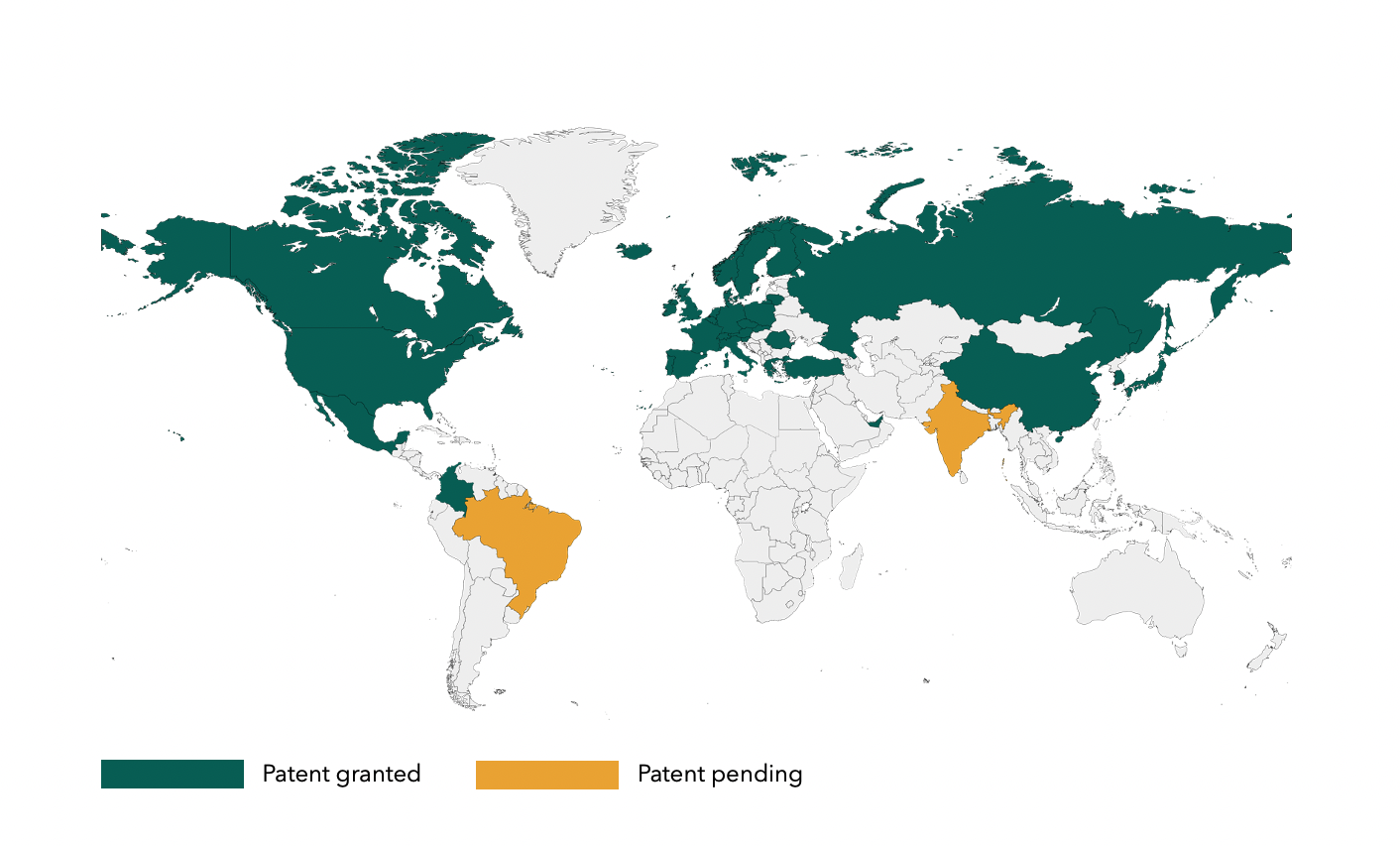
To further strengthen the platform, StemMedical has invested significantly in securing and protecting its key technology. More than 80% of the global market is covered by patents registered in 35 countries and pending in 3 countries
StemMedical patents cover the product composition of MSCs and adipose tissue, manufacturing of this composition, and its use as a cosmetic filler.
The coverage of the product composition is a product-by-process claim, meaning that the product is described by the manufacturing method (process):
A composition comprising ex vivo expanded primary culture (P0) adipose tissue-derived stem cells (ASCs) mixed with harvested fat tissue at a ratio of at least 20 x 106 to 20 x 107 ASCs/mL fat, wherein said ex vivo expanded primary culture (P0) adipose tissue-derived stem cells (ASCs) have been cultured in a growth medium consisting of Dulbecco’s modified Eagle's medium (DMEM) or alpha minimal essential medium (α-MEM), 1-5% penicillin‒streptomycin, 1-5 IU/mL preservative-free heparin and 2-20% pooled human platelet lysate (pHPL) and harvested in the primary passage (P0)