Platform

Proprietary best-in-class manufacturing platform for Mesenchymal Stem Cells
StemMedical’s core manufacturing competencies are focused on producing and expanding very high quantities of potent and affordable MSCs.
StemMedical can produce and harvest 12-16 billion ASCs from 100 ml of adipose tissue over 2 weeks using a scalable 3D cell culturing process instead of a manual non-scalable 2D cell process over several more weeks (as is currently industry standard).
Production takes place at our production facility outside Copenhagen. To expand capacity, blueprints have been made for a 2,500 sqm manufacturing site, designed for Good Manufacturing Practices (GMP) readiness, and with a planned capacity of over 7.000 batches per year. Work is currently in preparatory stage.
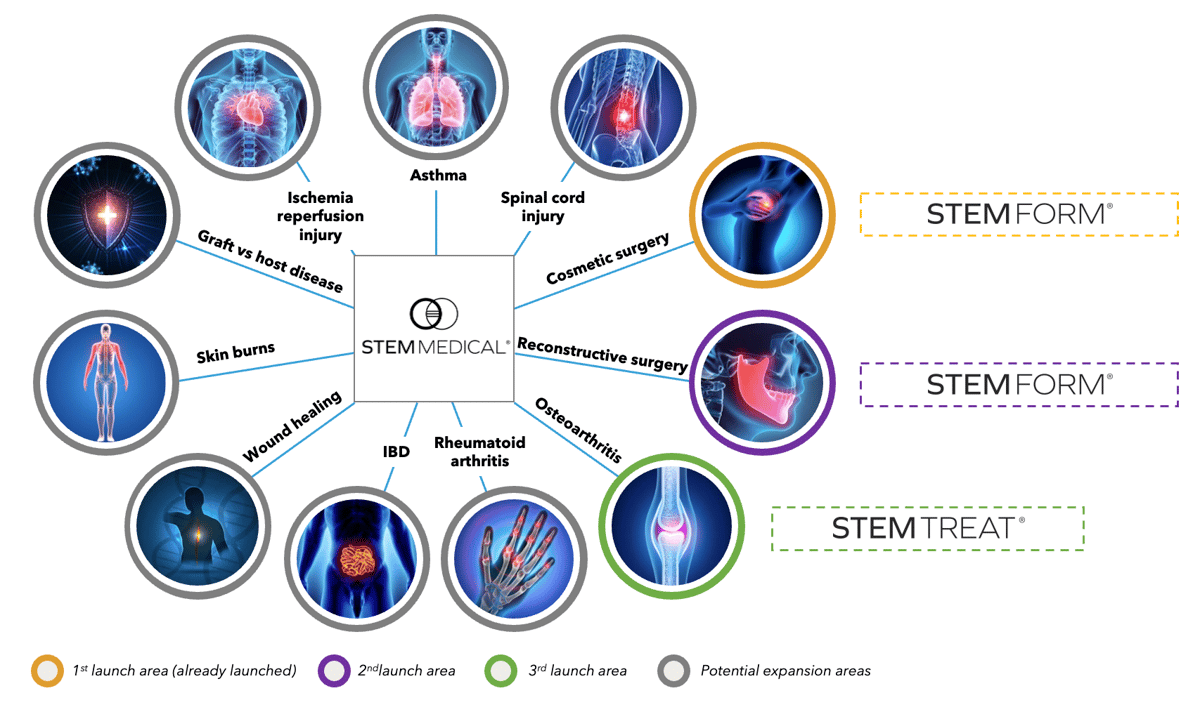
The StemMedical platform has several potential applications
StemMedical has overcome the 3 key challenges preventing effective stem cell-based therapies from reaching patients
Manufacturing
Produce enough cells at positive margin
(most struggle with producing enough cells even for trials)
Cell Therapy Space:
<0.1 billion MSCs per patient
StemMedical:
>12 billion MSCs per patient.
Clinical evidence
Demonstrate medical effect
(despite numerous trials, strong clinical results are in short order)
Cell Therapy Space:
Just 18 published results out of +1100 trials1
StemMedical:
Doubling of fat graft retention breakthrough published in Lancet.
1) As of 2020, and mainly in cardiology, osteoarthritis and neurology
Regulatory
Obtain regulatory clearance
(very few cell therapies approved)
Cell Therapy Space:
One MSC-based therapy approved in EU2,3,4 and zero in US2
StemMedical:
Several European countries has given regulatory clearance
2) As of May 2023 3) Alofisel (Takeda) 4 ~25 MSC-based therapy approved outside US/EU jurisdiction as of May 2022, typically just in single countries
